224x Filetype PDF File size 0.19 MB Source: www.ntnu.edu
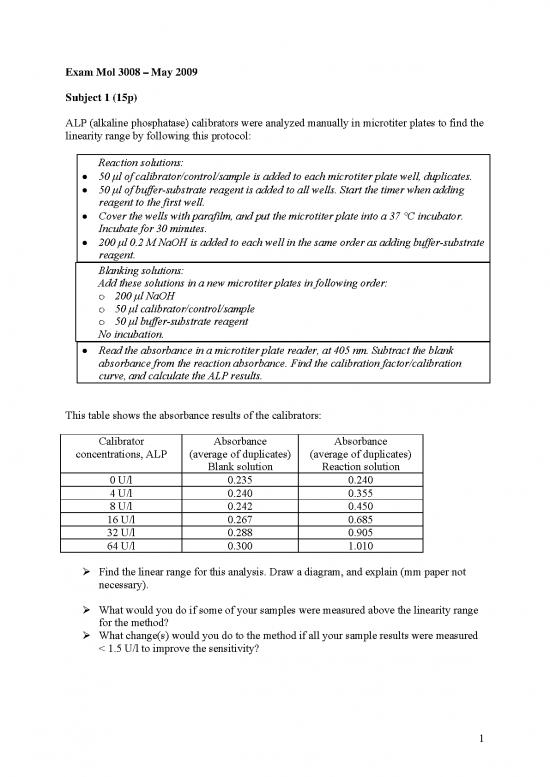
Exam Mol 3008 – May 2009
Subject 1 (15p)
ALP (alkaline phosphatase) calibrators were analyzed manually in microtiter plates to find the
linearity range by following this protocol:
Reaction solutions:
• 50 μl of calibrator/control/sample is added to each microtiter plate well, duplicates.
• 50 μl of buffer-substrate reagent is added to all wells. Start the timer when adding
reagent to the first well.
• Cover the wells with parafilm, and put the microtiter plate into a 37 °C incubator.
Incubate for 30 minutes.
• 200 μl 0.2 M NaOH is added to each well in the same order as adding buffer-substrate
reagent.
Blanking solutions:
Add these solutions in a new microtiter plates in following order:
o 200 μl NaOH
o 50 μl calibrator/control/sample
o 50 μl buffer-substrate reagent
No incubation.
• Read the absorbance in a microtiter plate reader, at 405 nm. Subtract the blank
absorbance from the reaction absorbance. Find the calibration factor/calibration
curve, and calculate the ALP results.
This table shows the absorbance results of the calibrators:
Calibrator Absorbance Absorbance
concentrations, ALP (average of duplicates) (average of duplicates)
Blank solution Reaction solution
0 U/l 0.235 0.240
4 U/l 0.240 0.355
8 U/l 0.242 0.450
16 U/l 0.267 0.685
32 U/l 0.288 0.905
64 U/l 0.300 1.010
¾ Find the linear range for this analysis. Draw a diagram, and explain (mm paper not
necessary).
¾ What would you do if some of your samples were measured above the linearity range
for the method?
¾ What change(s) would you do to the method if all your sample results were measured
< 1.5 U/l to improve the sensitivity?
1
Subject 2 (15p)
a) Describe the technique called western blot or immuno-blotting. Suggest strategies for how
to ensure that the signal you get represents the correct protein(s).
b) You would like to visualize the localization of two different proteins (antigen)
simultaneously by immuno-staining. What considerations would you have to do previous to
staining? What kind of controls could you do to ensure the specificity of your signal?
c) Antibodies are important tools in life science.
- What is the difference between a primary and secondary antibody?
- What is the difference between a native and denatured antigen?
Subject 3 (10p)
a) Outline schematically the most important parts in equipment used for High Performance
Liquid Chromatography (HPLC).
b) If your chromatogram has peaks that are not well separated from each other, how can you
improve the resolution in a HPLC analysis?
Subject 4 (10p)
a) Describe the fundamental principle of a confocal laser scanning microscope (confocal
microscope).
b) Discuss briefly the need of controls and relevant source of errors.
Subject 5 (5p)
Describe a simple enzyme-linked immunosorbent assay (ELISA). Describe how such an assay
now can be used to determine the amount of several antigens simultaneously in the same tube.
Subject 6 (5p)
When measuring protein concentrations with BioRad Protein Assay, the linearity range of the
method depends on what standard (calibrator) material you choose; an albumin standard or a
globulin standard. Explain why.
2
Multiple choice questions (only one cross for correct answer).
Correct answer gives 1 p
1.
Quantification of immunoreactive proteins may be performed by turbidity
measurements. Turbidity causes light scattering which involves
A A restricted range where Beer`s law could be applied
B Increased emission
C Reduced transmission
D Reduced emision which should be taken into account while calculating the results
2.
A competitive inhibitor competes with the substrate in binding to the enzyme.
This kind of inhibition causes
A Decreased Vmax and increased Km
B Increased Vmax and unchanged Km
C Decreased Km and unchanged Vmax
D Increased Km and unchanged Vmax
3.
A non-competitive inhibitor binds to the enzyme but does not interfere with the
catalytic site of the enzyme. This kind of inhibition
A Is always irreversible
B Is due to changes in configuration of the protein
C Is due to changes in conformation of the protein
D Is caused by a prostetic group
4.
In an electrophoresis system, using a native gel (e.g. agarose), proteins are separated
due to differences in total charge.
What decides the proteins’ total charge?
A The voltage
B The buffer’s pH
C The protein size
D The support medium
5.
In electrophoretic separation of proteins in an agarose gel, electroendosmotic flow
occurs. This is a buffer flow that may disturb protein migration in the gel.
Which of the following alternatives will reduce electroendosmotic flow?
A Applying less amount of sample
B Increasing the voltage
C Running the gel at low temperature
D Using a support medium with lower charge
3
6.
The image shows serum proteins separated in 1% agarose gel, 25 minutes at 100 Volts,
stained with Ponseau S protein stain. The separation of the proteins is not optimal.
+ ÷
What would you do to improve the separation?
A Increase the agarose concentration
B Run the electrophoresis at 75 V instead of 100
C Run the electrophoresis for 15 minutes instead of 25
D Use a lipid stain instead of a protein stain
7.
The schematic drawing shows separation of DNA fragments in a capillary
electrophoresis system, where the capillary is filled with a polymer.
The separation is based upon the following principle:
A Differences in current during the electrophoresis run
B Different charge of the DNA fragments
C Different molecule size of the DNA fragments
D Differences in electroendosmotic flow during the electrophoresis run
8.
Chromatography is often divided into gas chromatography and liquid chromatography.
For gas chromatography, which is the most correct characteristic: The mobile phase is a
gas and
A the stationary phase is a liquid
B the stationary phase is a solid
C the stationary phase is a liquid or a solid
D the samples to be separated are gases
E the samples to be separated are liquids
4
no reviews yet
Please Login to review.