218x Filetype XLS File size 0.15 MB Source: yoda.yale.edu
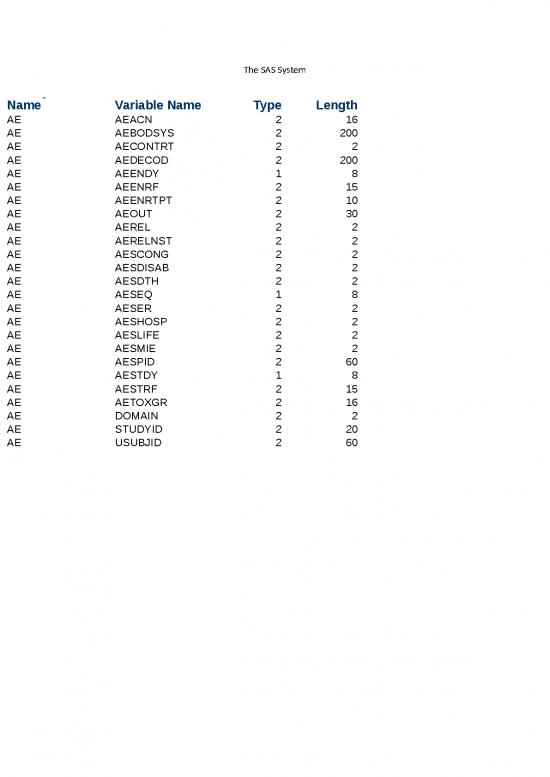
Sheet 1: AE
| Library Member Name | Variable Name | Variable Type | Variable Length | Variable Label | Variable Format | Observations in Data Set |
| AE | AEACN | 2 | 16 | Action Taken with Study Treatment | 19885 | |
| AE | AEBODSYS | 2 | 200 | Body System or Organ Class | 19885 | |
| AE | AECONTRT | 2 | 2 | Concomitant or Additional Trtmnt Given | 19885 | |
| AE | AEDECOD | 2 | 200 | Dictionary-Derived Term | 19885 | |
| AE | AEENDY | 1 | 8 | Study Day of End of Adverse Event | 19885 | |
| AE | AEENRF | 2 | 15 | End Relative to Reference Period | 19885 | |
| AE | AEENRTPT | 2 | 10 | End Relative to Reference Time Point | 19885 | |
| AE | AEOUT | 2 | 30 | Outcome of Adverse Event | 19885 | |
| AE | AEREL | 2 | 2 | Causality | 19885 | |
| AE | AERELNST | 2 | 2 | Relationship to Non-Study Treatment | 19885 | |
| AE | AESCONG | 2 | 2 | Congenital Anomaly or Birth Defect | 19885 | |
| AE | AESDISAB | 2 | 2 | Persist or Signif Disability/Incapacity | 19885 | |
| AE | AESDTH | 2 | 2 | Results in Death | 19885 | |
| AE | AESEQ | 1 | 8 | Sequence Number | 19885 | |
| AE | AESER | 2 | 2 | Serious Event | 19885 | |
| AE | AESHOSP | 2 | 2 | Requires or Prolongs Hospitalization | 19885 | |
| AE | AESLIFE | 2 | 2 | Is Life Threatening | 19885 | |
| AE | AESMIE | 2 | 2 | Other Medically Important Serious Event | 19885 | |
| AE | AESPID | 2 | 60 | Sponsor-Defined Identifier | 19885 | |
| AE | AESTDY | 1 | 8 | Study Day of Start of Adverse Event | 19885 | |
| AE | AESTRF | 2 | 15 | Start Relative to Reference Period | 19885 | |
| AE | AETOXGR | 2 | 16 | Standard Toxicity Grade | 19885 | |
| AE | DOMAIN | 2 | 2 | Domain Abbreviation | 19885 | |
| AE | STUDYID | 2 | 20 | Study Identifier | 19885 | |
| AE | USUBJID | 2 | 60 | Unique Subject Identifier | 19885 |
| Library Member Name | Variable Name | Variable Type | Variable Length | Variable Label | Variable Format | Observations in Data Set |
| CE | CECAT | 2 | 30 | Category for Clinical Event | 1230 | |
| CE | CEOCCUR | 2 | 1 | Clinical Event Occurrence | 1230 | |
| CE | CEPRESP | 2 | 1 | Clinical Event Pre-Specified | 1230 | |
| CE | CESEQ | 1 | 8 | Sequence Number | 1230 | |
| CE | CESTDY | Study Day of Start of Clinical Event | ||||
| CE | CETERM | 2 | 30 | Reported Term for the Clinical Event | 1230 | |
| CE | DOMAIN | 2 | 2 | Domain Abbreviation | 1230 | |
| CE | STUDYID | 2 | 20 | Study Identifier | 1230 | |
| CE | USUBJID | 2 | 60 | Unique Subject Identifier | 1230 |
| Library Member Name | Variable Name | Variable Type | Variable Length | Variable Label | Variable Format | Observations in Data Set |
| CM | CMCAT | 2 | 80 | Category for Medication | 35113 | |
| CM | CMDECOD | 2 | 200 | Standardized Medication Name | 35113 | |
| CM | CMDOSE | 1 | 8 | Dose per Administration | 35113 | |
| CM | CMDOSFRQ | 2 | 30 | Dosing Frequency per Interval | 35113 | |
| CM | CMDOSTXT | 2 | 10 | Dose Description | 35113 | |
| CM | CMDOSU | 2 | 20 | Dose Units | 35113 | |
| CM | CMENDY | 1 | 8 | Study Day of End of Medication | 35113 | |
| CM | CMENRTPT | 2 | 10 | End Relative to Reference Time Point | 35113 | |
| CM | CMOCCUR | 2 | 1 | CM Occurrence | 35113 | |
| CM | CMPRESP | 2 | 1 | CM Pre-Specified | 35113 | |
| CM | CMROUTE | 2 | 30 | Route of Administration | 35113 | |
| CM | CMSEQ | 1 | 8 | Sequence Number | 35113 | |
| CM | CMSPID | 2 | 50 | Sponsor-Defined Identifier | 35113 | |
| CM | CMSTDY | 1 | 8 | Study Day of Start of Medication | 35113 | |
| CM | CMSTRTPT | 2 | 10 | Start Relative to Reference Time Point | 35113 | |
| CM | DOMAIN | 2 | 2 | Domain Abbreviation | 35113 | |
| CM | STUDYID | 2 | 20 | Study Identifier | 35113 | |
| CM | USUBJID | 2 | 60 | Unique Subject Identifier | 35113 |
no reviews yet
Please Login to review.