393x Filetype XLSX File size 0.02 MB Source: www.gs1.org
Sheet 1: Sheet1
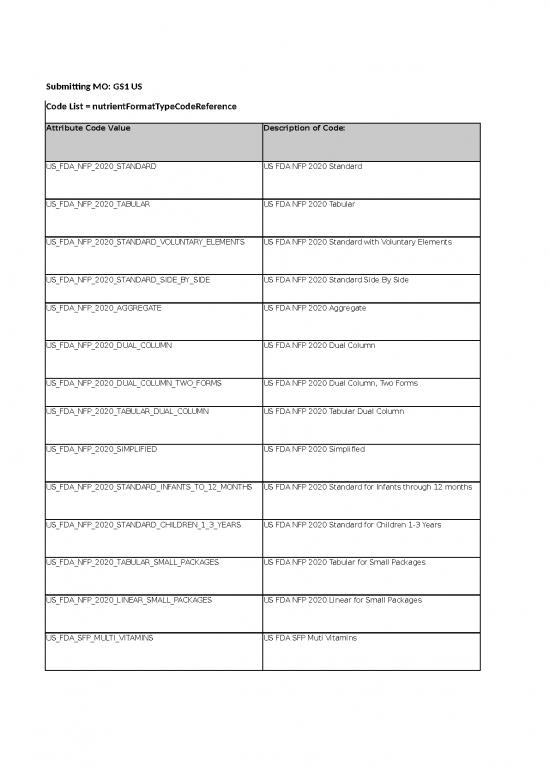
| Submitting MO: GS1 US | ||||
| Code List = nutrientFormatTypeCodeReference | ||||
| Attribute Code Value | Description of Code: | Definition in English | Definition Language 2 | Business Rule |
| US_FDA_NFP_2020_STANDARD | US FDA NFP 2020 Standard | Standard Vertical 21 CFR 101.9(d)(12) US FDA 2020 Food https://www.fda.gov/downloads/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/LabelingNutrition/UCM511964.pdf |
||
| US_FDA_NFP_2020_TABULAR | US FDA NFP 2020 Tabular | Tabular Format 21 CFR 101.9(d)(11)(iii) US FDA 2020 Food https://www.fda.gov/downloads/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/LabelingNutrition/UCM511964.pdf |
||
| US_FDA_NFP_2020_STANDARD_VOLUNTARY_ELEMENTS | US FDA NFP 2020 Standard with Voluntary Elements | Vertical Display Including Some Voluntary Nutrients 21 CFR 101.9(d)(12) US FDA 2020 Food https://www.fda.gov/downloads/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/LabelingNutrition/UCM511964.pdf |
||
| US_FDA_NFP_2020_STANDARD_SIDE_BY_SIDE | US FDA NFP 2020 Standard Side By Side | Standard Side By Side US FDA 2020 Food https://www.fda.gov/downloads/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/LabelingNutrition/UCM511964.pdf | ||
| US_FDA_NFP_2020_AGGREGATE | US FDA NFP 2020 Aggregate | Aggregate Display 21 CFR 101.9(d)(13)(ii) US FDA 2020 Food https://www.fda.gov/downloads/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/LabelingNutrition/UCM511964.pdf |
||
| US_FDA_NFP_2020_DUAL_COLUMN | US FDA NFP 2020 Dual Column | Dual Column Display, Per Serving and Per Container 21 CFR 101.9(e)(6)(i) US FDA 2020 Food https://www.fda.gov/downloads/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/LabelingNutrition/UCM511964.pdf |
||
| US_FDA_NFP_2020_DUAL_COLUMN_TWO_FORMS | US FDA NFP 2020 Dual Column, Two Forms | Dual Columns, Two Forms of the Same Food 21 CFR 101.9(e)(5) US FDA 2020 Food https://www.fda.gov/downloads/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/LabelingNutrition/UCM511964.pdf | ||
| US_FDA_NFP_2020_TABULAR_DUAL_COLUMN | US FDA NFP 2020 Tabular Dual Column | Tabular Dual Column Display 21 CFR 101.9(e)(6)(ii) US FDA 2020 Food https://www.fda.gov/downloads/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/LabelingNutrition/UCM511964.pdf |
||
| US_FDA_NFP_2020_SIMPLIFIED | US FDA NFP 2020 Simplified | Simplified Display 21 CFR 101.9(f) US FDA 2020 Food https://www.fda.gov/downloads/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/LabelingNutrition/UCM511964.pdf |
||
| US_FDA_NFP_2020_STANDARD_INFANTS_TO_12_MONTHS | US FDA NFP 2020 Standard for Infants through 12 months | Infants through 12 Months of Age 21 CFR 101.9(j)(5)(ii)(B) US FDA 2020 Food https://www.fda.gov/downloads/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/LabelingNutrition/UCM511964.pdf |
||
| US_FDA_NFP_2020_STANDARD_CHILDREN_1_3_YEARS | US FDA NFP 2020 Standard for Children 1-3 Years | Children 1-3 Years 21 CFR 101.9(j)(5)(iii)(A) US FDA 2020 Food https://www.fda.gov/downloads/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/LabelingNutrition/UCM511964.pdf |
||
| US_FDA_NFP_2020_TABULAR_SMALL_PACKAGES | US FDA NFP 2020 Tabular for Small Packages | Tabular Display for Small or Intermediate-Sized Packages 21 CFR 101.9(j)(13)(ii)(A)(1) US FDA 2020 Food https://www.fda.gov/downloads/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/LabelingNutrition/UCM511964.pdf |
||
| US_FDA_NFP_2020_LINEAR_SMALL_PACKAGES | US FDA NFP 2020 Linear for Small Packages | Linear Display for Small or Intermediate-Sized Packages 21 CFR 101.9(j)(13)(ii)(A)(2) US FDA 2020 Food https://www.fda.gov/downloads/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/LabelingNutrition/UCM511964.pdf |
||
| US_FDA_SFP_MULTI_VITAMINS | US FDA SFP Muti Vitamins | Multivitamins 21 CFR 101.9 US FDA Supplemental Facts https://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/DietarySupplements/ucm070597.htm |
||
| US_FDA_SFP_MULTI_VITAMINS_IN_PACKAGES | US FDA SFP Multi Vitamins in Packages | Multivitamins in Packs 21 CFR 101.9 US FDA Supplemental Facts https://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/DietarySupplements/ucm070597.htm |
||
| US_FDA_SFP_MULTI_VITAMINS_PER_DAY_PER_SERVING | US FDA SFP Muti Vitamins Per Day-Per Serving | Per Day- Per Serving 21 CFR 101.9 US FDA Supplemental Facts https://www.ecfr.gov/cgi-bin/retrieveECFR?gp=1&SID=4bf49f997b04dcacdfbd637db9aa5839&ty=HTML&h=L&mc=true&n=pt21.2.101&r=PART#se21.2.101_19 |
||
| US_FDA_SFP_STANDARD | US FDA SFP Standard | Standard 21 CFR 101.9 US FDA Supplemental Facts https://www.ecfr.gov/cgi-bin/retrieveECFR?gp=1&SID=4bf49f997b04dcacdfbd637db9aa5839&ty=HTML&h=L&mc=true&n=pt21.2.101&r=PART#se21.2.101_19 |
||
| US_FDA_NFP_PRE_2020_AGGREGATE | US FDA NFP Pre-2020 Aggregate | Aggregate Display 21 CFR 101.9 US FDA Pre-2020 Food https://www.ecfr.gov/cgi-bin/retrieveECFR?gp=1&SID=4bf49f997b04dcacdfbd637db9aa5839&ty=HTML&h=L&mc=true&n=pt21.2.101&r=PART#se21.2.101_19 |
||
| US_FDA_NFP_PRE_2020_STANDARD | US FDA NFP Pre-2020 Standard | Standard Vertical 21 CFR 101.9 US FDA Pre-2020 Food https://www.ecfr.gov/cgi-bin/retrieveECFR?gp=1&SID=4bf49f997b04dcacdfbd637db9aa5839&ty=HTML&h=L&mc=true&n=pt21.2.101&r=PART#se21.2.101_19 |
||
| US_FDA_NFP_PRE_2020_TABULAR | US FDA NFP Pre-2020 Tabular | Tabular Display for Small or Intermediate-Sized Packages 21 CFR 101.9 US FDA Pre-2020 Food https://www.ecfr.gov/cgi-bin/retrieveECFR?gp=1&SID=4bf49f997b04dcacdfbd637db9aa5839&ty=HTML&h=L&mc=true&n=pt21.2.101&r=PART#se21.2.101_19 |
||
| US_FDA_NFP_PRE_2020_DUAL_COLUMN | US FDA NFP Pre-2020 Dual Column | Dual Columns, Two Forms of the Same Food 21 CFR 101.9 US FDA Pre-2020 Food https://www.ecfr.gov/cgi-bin/retrieveECFR?gp=1&SID=4bf49f997b04dcacdfbd637db9aa5839&ty=HTML&h=L&mc=true&n=pt21.2.101&r=PART#se21.2.101_19 |
||
| US_FDA_NFP_PRE_2020_LINEAR | US FDA NFP Pre-2020 Linear | Linear 21 CFR 101.9 US FDA Pre-2020 Food https://www.ecfr.gov/cgi-bin/retrieveECFR?gp=1&SID=4bf49f997b04dcacdfbd637db9aa5839&ty=HTML&h=L&mc=true&n=pt21.2.101&r=PART#se21.2.101_19 |
||
| Summary of Exemption | Regulation # |
| *Manufactured by small businesses | 21 CFR 101.9(j)(1) and 101.9(j)(18) |
| *Food served in restaurants, etc. or delivered to homes ready for immediate consumption | 21 CFR 101.9(j)(2) |
| *Delicatessen-type food, bakery products and confections that are sold directly to consumers from the location where prepared | 21 CFR 101.9(j)(3) |
| *Foods that provide no significant nutrition such as instant coffee (plain, unsweetened) and most spices | 21 CFR 101.9(j)(4) |
| Infant formula, and infant and junior foods for children up to 4 years of age (modified label provisions for these categories) | 21 CFR 101.9(j)(5) and 101.9(j)(7) |
| Dietary supplements (must comply with 21 CFR 101.36) | 21 CFR 101.9(j)(6) |
| Medical foods | 21 CFR 101.9(j)(8) |
| Bulk foods shipped for further processing or packaging before retail sale | 21 CFR 101.9(j)(9) |
| Fresh produce and seafood (a voluntary nutrition labeling program covers these foods through the use of the appropriate means such as shelf labels, signs, and posters) | 21 CFR 101.9(j)(10) and 101.45 |
| Packaged single-ingredient fish or game meat may be labeled on basis of 3-ounce cooked portion (as prepared). Custom-processed fish and game are exempt from nutrition labeling. | 21 CFR 101.9(j)(11) |
| Certain egg cartons (nutrition information inside lid or on insert in carton) | 21 CFR 101.9(j)(14) |
| Packages labeled "This unit not labeled for retail sale" within multiunit package, and outer wrapper bears all required label statements | 21 CFR 101.9(j)(15) |
| Self-service bulk foods--nutrition labeling by placard, or on original container displayed clearly in view | 21 CFR 101.9(a)(2) and 101.9(j)(16) |
| Donated food that is given free (not sold) to the consumer. | You are not required to put Nutrition Facts labels on donated food unless the donated food is later placed on sale (the law applies only to food that is "offered for sale") -- 21 CFR 101.9(a) |
| Game meats may provide required nutrition information or labeling in accordance with 21 CFR 101.9(a)(2). | 21 CFR 101.9(j)(12) |
no reviews yet
Please Login to review.