268x Filetype PDF File size 0.21 MB Source: web.mst.edu
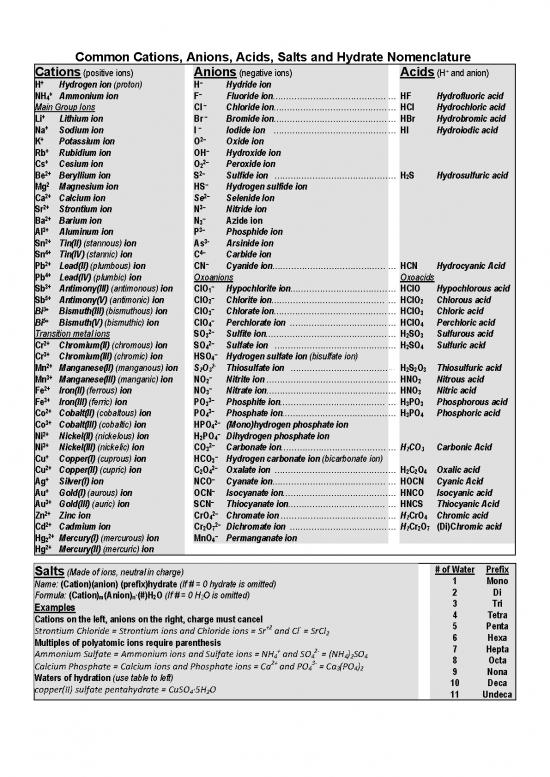
Common Cations, Anions, Acids, Salts and Hydrate Nomenclature
Cations (positive ions) Anions (negative ions) Acids (H+ and anion)
H+ Hydrogen ion (proton) H– Hydride ion
NH+ Ammonium ion F– Fluoride ion…………………………………… … HF Hydrofluoric acid
4
–
Main Group Ions Cl Chloride ion…………………………………… … HCl Hydrochloric acid
+ –
Li Lithium ion Br Bromide ion…………………………………… … HBr Hydrobromic acid
+ –
Na Sodium ion I Iodide ion …………………………………… … HI Hydroiodic acid
K+ Potassium ion O2– Oxide ion
+ –
Rb Rubidium ion OH Hydroxide ion
+ 2–
Cs Cesium ion O2 Peroxide ion
2+ 2–
Be Beryllium ion S Sulfide ion …………………………………… … H2S Hydrosulfuric acid
2 –
Mg Magnesium ion HS Hydrogen sulfide ion
2+ 2–
Ca Calcium ion Se Selenide Ion
2+ 3–
Sr Strontium ion N Nitride ion
2+ –
Ba Barium ion N3 Azide ion
3+ 3–
AI Aluminum ion P Phosphide ion
2+ 3-
Sn Tin(II) (stannous) ion As Arsinide ion
4+ 4–
Sn Tin(IV) (stannic) ion C Carbide ion
2+ –
Pb Lead(II) (plumbous) ion CN Cyanide ion…………………………………… … HCN Hydrocyanic Acid
4+
Pb Lead(IV) (plumbic) ion Oxoanions Oxoacids
3+ –
Sb Antimony(III) (antimonous) ion CIO1 Hypochlorite ion……………………………… … HCIO Hypochlorous acid
5+ –
Sb Antimony(V) (antimonic) ion CIO2 Chlorite ion…………………………………… … HCIO2 Chlorous acid
3+ –
Bi Bismuth(III) (bismuthous) ion CIO3 Chlorate ion…………………………………… … HCIO3 Chloric acid
5+ –
Bi Bismuth(V) (bismuthic) ion ClO4 Perchlorate ion ……………………………… … HClO4 Perchloric acid
Transition metal ions SO32– Sulfite ion……………………………………… … H2SO3 Sulfurous acid
2+ 2–
Cr Chromium(II) (chromous) ion SO4 Sulfate ion …………………………………… … H2SO4 Sulfuric acid
3+ –
Cr Chromium(III) (chromic) ion HSO4 Hydrogen sulfate ion (bisulfate ion)
2+ 2- …
Mn Manganese(II) (manganous) ion S2O3 Thiosulfate ion ……………………………… H2S2O3 Thiosulfuric acid
3+ –
Mn Manganese(III) (manganic) ion NO2 Nitrite ion ……………………………………… … HNO2 Nitrous acid
2+ –
Fe Iron(II) (ferrous) ion NO3 Nitrate ion……………………………………… … HNO3 Nitric acid
3+ 3–
Fe Iron(III) (ferric) ion PO3 Phosphite ion………………………………… … H3PO3 Phosphorous acid
2+ 3–
Co Cobalt(II) (cobaltous) ion PO4 Phosphate ion………………………………… … H3PO4 Phosphoric acid
3+ 2–
Co Cobalt(III) (cobaltic) ion HPO4 (Mono)hydrogen phosphate ion
2+ –
Ni Nickel(II) (nickelous) ion H2PO4 Dihydrogen phosphate ion
3+ 2–
Ni Nickel(III) (nickelic) ion CO3 Carbonate ion………………………………… … H2CO3 Carbonic Acid
+ –
Cu Copper(l) (cuprous) ion HCO3 Hydrogen carbonate ion (bicarbonate ion)
2+ 2–
Cu Copper(II) (cupric) ion C2O4 Oxalate ion …………………………………… … H2C2O4 Oxalic acid
+ –
Ag Silver(I) ion NCO Cyanate ion…………………………………… … HOCN Cyanic Acid
+ –
Au Gold(I) (aurous) ion OCN Isocyanate ion………………………………… … HNCO Isocyanic acid
3+ –
Au Gold(III) (auric) ion SCN Thiocyanate ion……………………………… … HNCS Thiocyanic Acid
2+ 2–
Zn Zinc ion CrO4 Chromate ion ………………………………… … H2CrO4 Chromic acid
2+ 2–
Cd Cadmium ion Cr O Dichromate ion ……………………………… … H Cr O (Di)Chromic acid
2 7 2 2 7
2+ –
Hg Mercury(I) (mercurous) ion MnO Permanganate ion
2 4
2+
Hg Mercury(II) (mercuric) ion
Salts (Made of ions, neutral in charge) # of Water Prefix
Name: (Cation)(anion) (prefix)hydrate (If # = 0 hydrate is omitted) 1 Mono
Formula: (Cation)m(Anion)n·(#)H2O (If # = 0 H2O is omitted) 2 Di
Examples 3 Tri
Cations on the left, anions on the right, charge must cancel 4 Tetra
+2 ‐ 5 Penta
Strontium Chloride = Strontium ions and Chloride ions = Sr and Cl = SrCl
2 6 Hexa
Multiples of polyatomic ions require parenthesis 7 Hepta
Ammonium Sulfate = Ammonium ions and Sulfate ions = NH + and SO 2‐ = (NH ) SO
4 4 4 2 4 8 Octa
2+ 3‐
Calcium Phosphate = Calcium ions and Phosphate ions = Ca and PO4 = Ca3(PO4)2 9 Nona
Waters of hydration (use table to left) 10 Deca
copper(II) sulfate pentahydrate = CuSO4∙5H2O 11 Undeca
Common Covalent Binary Inorganic Compounds
# of atoms Prefix Common Examples (element closest to fluorine goes on right)
1 Mono H2 Hydrogen N2 Nitrogen
2 Di O Oxygen NH Ammonia
2 3
3 Tri O3 Ozone NO Nitrogen monoxide (Nitric Oxide)
4 Tetra H2O Water (Dihydrogen Monoxide) NO2 Nitrogen dioxide
5 Penta F2 Fluorine N2O Dinitrogen monoxide (Nitrous oxide)
6 Hexa HF Hydrogen fluoride N2O2 Dinitrogen dioxide
7 Hepta Cl Chlorine N O Dinitrogen tetroxide
2 2 4
8 Octa HCl Hydrogen chloride CO Carbon monoxide
9 Nona Br Bromine CO Carbon dioxide
2 2
10 Deca I Iodine CCl Carbon tetrachloride
2 4
Organic Nomenclature and Symbolism
(Other group prefixes)(longest chain prefix)(highest bond root)(most important group suffix)
Bond Order Name Drawn Root Formula Carbon has 4 bonds Carbon Chain Prefix
**
1 Single C-C ane C H In formula: # Systematic Common
n 2n+2
2 Double C=C ene C H Groups -1 H 1 Methyl Formyl
n 2n
3 Triple C≡C yne CnH2n-2 Other C-C bonds -2 H 2 Ethyl Acetyl
3 Propyl Propionyl
Group Name Drawn Prefix Suffix 4 Butyl Butyryl
Amine -NH Amino amine 5 Pentyl Valeryl
2
Ammonium ion -NH + ammonium ion 6 Hexyl Caproyl
3
Carboxylic acid* Carboxyl oic acid 7 Heptyl Enanthyl
or -COOH or -CO2H 8 Octyl Caprylyl
Carboxylate ion* oate ion 9 Nonyl Pelargonyl
or -COO- or -CO2- 10 Decyl Capryl
Alcohol -OH Hydroxy ol Drop ‘yl’ from prefix for longest chain
-F Fluoro
-Cl Chloro *Include carbon in chain prefix
Halogen -Br Bromo
**
-I Iodo Don’t use bond root (names only ane)
Drop ‘o’ from carboxyl groups
Aromatic or C H or -Ф or -Ph Phenyl (‘ic’ and ‘ate’)
6 5
Examples
Name Formula Systematic Name Common Name Formula Name Formula
Methane CH Methanoic acid Formic acid HCOH 1,2-Dichloroethane C H Cl
4 2 2 4 2
Ethane C H Ethanoic acid Acetic acid CHCOH Methylamine CHNH
2 6 3 2 3 2
Propane C H Propanoic acid Propionic acid C H CO H Methylammonium ion CHNH+
3 8 2 5 2 3 3
Butane C H Butanoic acid Butyric acid C H CO H 1,3-butadiene C H
4 10 3 7 2 4 6
Pentane C H Pentanoic acid Valeric acid C H CO H Hydroxyethanoic acid HOCH CO H
5 12 4 9 2 2 2
Methanol CHOH Methanoate ion Formate ion HCO- Phenol C H OH
3 2 6 5
Ethanol C H OH Ethanoate ion Acetate ion CHCO-
2 5 3 2
Propanol C3H7OH Propanoate ion Propionate ion C2H5CO2- Special Names Formula
Butanol C4H9OH Butanoate ion Butyrate ion C3H7CO2- Benzene C6H6
Pentanol C H OH Pentanoate ion Valerate ion C H CO - Toluene C H CH
5 11 4 9 2 6 5 3
Most Common Formula Representations (All represent ethanol)
Example C H O CHCHOH
2 6 3 2
Name Molecular Formula Condensed Molecular Structural Formula Line Formula
Formula
no reviews yet
Please Login to review.