240x Filetype PDF File size 0.73 MB Source: qe2.sch.im
iGCSE Scheme of work: Year 9: Chemistry
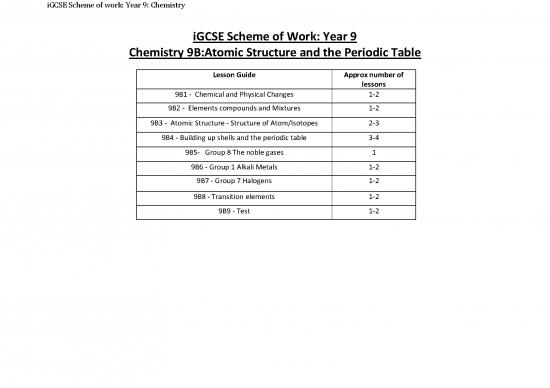
iGCSE Scheme of Work: Year 9
Chemistry 9B:Atomic Structure and the Periodic Table
Lesson Guide Approx number of
lessons
9B1 - Chemical and Physical Changes 1-2
9B2 - Elements compounds and Mixtures 1-2
9B3 - Atomic Structure - Structure of Atom/Isotopes 2-3
9B4 - Building up shells and the periodic table 3-4
9B5- Group 8 The noble gases 1
9B6 - Group 1 Alkali Metals 1-2
9B7 - Group 7 Halogens 1-2
9B8 - Transition elements 1-2
9B9 - Test 1-2
iGCSE Scheme of work: Year 9: Chemistry
QE2 No of Syllabus Points / Learning Suggested Resources/practicals etc Requisition Literacy PLTS
lessons objectives
9B1 1-2 ● C3.1.1 Identify physical and ● ‘C.3 Physical and Chemical Changes’ Powerpoint (good quality) Ignition tubes with Write Self
chemical changes and with Iron filings and sulfur experiment (nice experiment) which sulphur, iron and a practical manag
understand the differences also covers elements compounds mixtures (below) with mixture of sulphur ers
between them worksheet - students like smashing ignition tubes! (in a and iron, magnets Key
● C3.1.2 (supplementary only) newspaper with goggles!) hammer terms Reflect
Understand that some ● Alternatively ‘Chemical and physical changes’ powerpoint of newspaper (one ive
chemical reactions can be lower risk experiments that can be set up as a circus - tables between 2) learner
reversed and experiment guides included in powerpoint that can be s
printed (includes opportunity for some practical skills work with or
peer assessment in a second lesson)
● C.3 Physical and chemical changes word doc as a homework Vinegar, sugar,
good for self or peer assessment when back in class. bicarbonate of
● ‘Physical or Chemical changes?’powerpoint quiz (decent for a soda, magnesium
plenary) ribbon, lime
● Chem for you Chem & Phys Changes (Expt 2.4) water, straws
● Essential science chem book 7.1 IS NO USE FOR THIS
SYLLABUS POINT or
● Anhydrous Copper sulphate powder - add few drops of water
with a pipette (turns blue) then heat in an evaporating basin to CFY Expt 2.4
return to white powder (fine as a class practical between 2)
or
Anhydrous Copper
sulphate powder
9B2 1-2 ● C3.2.1 Describe the ● Elements compound mixtures powerpoint explaining the Definitio SM
differences between differences, covers metals and non metals too ns
elements compounds ● Quiz for elements compounds and mixtures on powerpoint
and mixtures and (good plenary)
between metals and ● ecm.jpg elements compound and mixtures worksheet (good
non metals plenary)
● C3.2.2 Define the term ● The sulphur and iron filings in 9B1 section does a good job
solvent, solute, solution of covering this syllabus point
and concentration ● Essentials book pages 28-29
(covered in 9A SOW,
doesn't really fit here)
iGCSE Scheme of work: Year 9: Chemistry
9B3 2-3 ● C3.3.1 Describe the ● Particles in an atom ppt could be used as an introduction to Atom Models
structure of the atom in atomic structure
terms of a central ● Atomic structure worksheet (as it says, good for
nucleus containing consolidation) referred to in above ppt
protons neutrons and ● Particles in an atom doc - independent task with reading for
shells of electrons a literacy focus would only use to introduce topic/cover
● C3.3.3 State the charges lesson
of approximate charges ● Atom models are very useful here
and relative atomic ● Boardworks powerpoint is thorough and has dedicated
masses of of protons worksheets attached and covers all the points here
electrons and neutrons (including isotopes) and leads into group properties a little
● C3.3.4 Define the term ● Isotopes ppt - low ability simple explanation
proton number as the ● Isotopes - mini plenary/starter on isotopes
number of protons in ● Isotopes and relative atomic mass worksheets and ppt - nice
the nucleus stretch activity to show higher ability students why Masses
● C3.3.5 define and use on periodic table are not round numbers. Nice homework
nucleon number as the task included.
total mass of protons ● Essentials Book pages 22-23
and neutrons in the
nucleus
● C3.3.7 Define isotopes
as atoms of the same
element that have the
same proton number
but a different nucleon
number
● C3.3.8 (supplementary
only)Understand that
isotopes have the same
properties as they have
the same number of
electrons in the outer
shell
9B4 2-3 ● C3.3.2 Describe the ● ‘Electron arrangement (brdwrks).ppt’ does this well with Atom models
build up of electrons in associated worksheet
shells and understand ● ‘Electron Arrangement’ powerpoint only covers electron
the significance of the configurations and would be best done with the atom
noble gas structure (see models - good for low ability introduction
iGCSE Scheme of work: Year 9: Chemistry
9B5) ● Electron configuration - very basic worksheet for intro /low
● C3.3.6 Use proton ability plenary
number and the and the ● Electron configuration homework - good plenary/homework
simple structure of exercise
atoms to to explain the ● Electron configuration pdf - great task to consolidate
basis of the periodic electron arrangement and start to make links between
table atomic structure and the periodic table
● C9.1.1 Describe the ● Electron Configuration post-it activity - group activity could
periodic table as a be done on post-its instructions could be given card at a
method of classifying time till they see the link between electron configuration
elements and predicting and periodic table - serves same purpose as the periodic
properties of elements table but may be a bit more independent
● C9.2.1 Describe the ● Groups of the periodic table worksheet - good to
trend from metallic to consolidate and link into the different groups and periods.
nonmetallic across a could be a decent cover lesson - quite long though and may
period lose lower ability groups
● C9.2.2 Describe the ● Could do history of the periodic table display/comparison
relationship between with Mendelevs table to make links
group number and ● Essential Chemistry book pages 26-27
number of electrons in ● Essential Chemistry Book Pages 152-153
the outer shell and ● ‘Essential Chemistry’ book page 30 for metals
metallic/non metallic
character
9B5 1 ● C9.5.1 Describe the ● Noble Gases Brdworks
noble gases in group 8 ● Noble Gases ppt with worksheet
as being unreactive ● Could link to air in 9A - as highly unreactive have a presence
mono-atomic gases and in air
explain this in terms of ● ‘Oh the humanity!’ you could watch the hindenburg video
electronic structure on you tube to show being inert can be a good thing!
● C9.5.2 State the uses of https://www.youtube.com/watch?v=CgWHbpMVQ1U
noble gases as in ● Neon or argon lamp could be used
providing an inert ● Essential Chemistry 158-159 (very dull)
atmosphere (argon
lamps, helium for
balloons)
no reviews yet
Please Login to review.